References
Podiatrist intervention could reduce the incidence of foot ulcers in patients with diabetes: a hospital survey in China
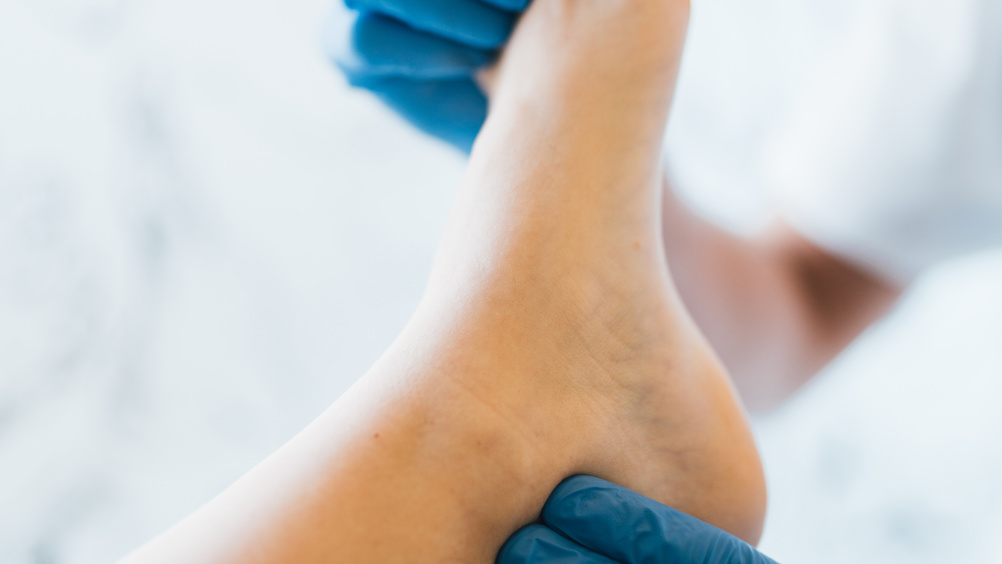
Abstract
Objective:
This study aimed to evaluate the effectiveness of podiatrists in preventing diabetic foot ulcers (DFUs) in China.
Method:
The study was a prospective investigation. A total of 300 patients were enrolled from May 2016 to May 2018 in Handan Central Hospital, China. All patients who participated in this study had been diagnosed with type 2 diabetes, according to the International Classification of Diseases (ICD-10). All participants underwent our survey, which included basic patient data and information about DFUs. The patients were followed for one year, during which time they received appropriate intervention from podiatrists, including lifestyle guidance, callus resection, tinea grinding and ingrown nail correction. At the end of the year all the patients were surveyed again. The data before and after the year were statistically compared.
Results:
The results showed that the incidence of DFUs in patients with diabetes was significantly decreased after one year of intervention from podiatrists (20.7% versus 6.7%, p<0.001). Additionally, there was a negative correlation between the number of intervention visits and the number of DFU occurrences (Spearman correlation coefficient: –0.496, p<0.001). Furthermore, we found that 68 patients with a history of DFUs or amputation had an obviously reduced incidence of DFUs after intervention by a podiatrist (89.7% versus 27.9%, p<0.001). We also investigated other foot risk factors in all participants, such as limb neuropathy (76.3%), lower extremity vascular disease (65.7%) and foot paralysis (43.7%).
Conclusion:
The results of this study help in understanding the situation of patients with diabetes in China and to prove that standardised podiatrist intervention has an important role in inhibiting the occurrence and development of DFUs.
Diabetic foot ulcers (DFUs), which are one of the widespread and serious complications in patients with diabetes, have been defined as ulcers on the feet associated with infection, destruction of the deep tissues, neurological abnormalities and peripheral vascular disease (PVD) in the lower limb.1 DFUs are a common reason for hospital admissions among patients with diabetes and are a major factor reducing their quality of life.2,3 DFUs increase patients' morbidity, mortality and healthcare expenditure.4,5 The lifetime incidence of foot ulcers in patients with diabetes is 34%.6 More than half of nontraumatic lower-extremity amputations have been related to DFU infections, and 85% of patients with diabetes and with amputations had DFUs.7,8 Patients with diabetes and with amputation because of DFUs have a mortality rate of >70% within five years.7 Statistical data in Taiwan from 2001–2015 showed that 20–30% of hospitalisation fees were caused by DFU and 18.6% of patients with DFUs underwent amputation.9
Register now to continue reading
Thank you for visiting Journal of Wound Care's Silk Road Supplement and reading some of our peer-reviewed resources for healthcare professionals across Asia. To read more, please register today.
