Maggot infestations have long been associated with improved wound healing by clinicians throughout history. Dominique Larrey, who worked as a battlefield surgeon in the Napoleonic Wars, noted that maggot-infested wounds tended to heal more rapidly, and observed that the maggots fed only on dead and devitalised tissue while leaving healthy tissue intact.1 Despite these observations, the deliberate introduction of maggots into infected wounds did not occur until the early twentieth century, when William Baer, the Professor of Orthopaedic Surgery at the Johns Hopkins School of Medicine in Maryland, US, pioneered the use of sterile maggots in the treatment of infected wounds and osteomyelitis with significant success.2
Following an initial wave of tetanus and gas gangrene in some patients treated with maggot therapy, Baer subsequently developed a method of sterilisation to reduce the risk of secondary infections from microorganisms present on the surface of non-sterile maggots.3 Maggot debridement therapy (MDT) proceeded to see ensuing utilisation within the treatment of wounds, only fading in popularity after the Second World War due to the discovery and widespread use of antibiotics.4 However, the increase in the prevalence of antibiotic-resistant strains in wound infections has led to a resurgence in maggot therapy since the 1980s.4
Maggot therapy was first introduced to Malaysia in 2003 at Lumut Navy Hospital, Perak, with the successful treatment of hard-to-heal diabetic wounds using the larvae of the blowfly Lucilia cuprina.5 The Australian sheep blowfly Lucilia cuprina is endemic to Southeast Asia and is a close relative of the common green bottle fly Lucilia sericata, of which the larvae has been widely used in maggot debridement therapy (MDT) in the West. The sterilisation of Lucilia cuprina eggs and first-instar larvae were established by the Institute for Medical Research (IMR) under the Ministry of Health of Malaysia. A number of clinical trials in Malaysia have shown MDT to be efficacious in the treatment of hard-to-heal wounds of various aetiology, being noninferior to conventional debridement while reducing hospital ward stay time and amputation rates.6
Our previous study involving nine patients treated with MDT demonstrated successful debridement with <5% of the wounds being covered by slough or necrotic tissue for six of these patients; whereas the remaining three patients had a ≥40% reduction in slough over their wound.7
This current study seeks to validate our findings with a larger patient population and further evaluate the efficacy of MDT in the treatment of hard-to-heal wounds.
Method
Participants were recruited via convenient sampling from patients attending the Wound Care Clinic at Kuala Lumpur General Hospital for diabetic foot ulcers (DFUs). Inclusion and exclusion criteria are given below.
Inclusion criteria:
- Hard-to-heal DFUs with failure to heal >12 weeks from onset, but of <12 months' duration
- Presence of slough, necrosis, eschar or inflammation, with or without infection.
Exclusion criteria:
- Peripheral vascular disease with an ankle–brachial pressure index (ABPI) <0.5
- Presence of ischaemia of the affected limb
- Wounds secondary to hard-to-heal venous insufficiency.
Patients with hard-to-heal venous ulcers were excluded from the study although many such wounds had a degree of overlap with diabetic aetiology. This was done as the compression dressings used to treat these wounds would also kill any maggots in the wound bed, hence invalidating the use of MDT for these patients.
Written consent was obtained before enrolment for both medical photography as well as commencement of MDT, and for the use in educational purposes and publications. Parameters assessed during the study period were: wound size; wound bed tissue; and pain score as measured with the visual analogue scale (VAS).
Maggots adhered to a cage dressing of sterile gauze were applied to the wound bed, with a standardised coverage of 10 first-instar maggots per 1cm2 of wound surface area. Washout and reassessment of the wound was conducted 72 hours after application, with re-application of first-instar maggots as needed.
The treatment endpoint was either successful wound healing or failure of the MDT, defined as absence of improvement following three consecutive applications of maggots as described above. MDT was then stopped for these patients and other methods of debridement were carried out as appropriate.
All patients included in the study had pre-existing subcutaneous insulin therapy, with regular monitoring of capillary blood to ensure optimal diabetic control.
Results
A total of 30 patients took part in this study (patient characteristics and location of wound are shown in Table 1), of whom 29 (96.6%) experienced maximum debridement of the wound surface area (defined as ≤5% coverage of the wound surface area by slough or necrotic tissue at the end of MDT). Only one patient (Case 6) had 10% of slough tissue at the end of MDT. Additionally, reduction in wound-related pain was reported across the study period.
Table 1. Patient characteristics and wound location
| Case number | Age, years | Sex | Wound location |
|---|---|---|---|
| 1 | 81 | Male | Foot |
| 2 | 71 | Male | Abdomen |
| 3 | 65 | Male | Leg |
| 4 | 59 | Male | Foot |
| 5 | 51 | Female | Foot |
| 6 | 57 | Male | Foot |
| 7 | 65 | Male | Leg |
| 8 | 76 | Male | Foot |
| 9 | 53 | Female | Foot |
| 10 | 57 | Male | Foot |
| 11 | 59 | Male | Foot |
| 12 | 47 | Male | Foot |
| 13 | 44 | Female | Leg |
| 14 | 65 | Male | Foot |
| 15 | 29 | Male | Foot |
| 16 | 70 | Male | Foot |
| 17 | 86 | Female | Foot |
| 18 | 60 | Female | Foot |
| 19 | 67 | Male | Foot |
| 20 | 69 | Female | Foot |
| 21 | 57 | Male | Foot |
| 22 | 63 | Male | Foot |
| 23 | 42 | Female | Foot |
| 24 | 70 | Female | Foot |
| 25 | 61 | Male | Leg |
| 26 | 69 | Male | Foot |
| 27 | 66 | Male | Foot |
| 28 | 71 | Male | Foot |
| 29 | 57 | Female | Foot |
| 30 | 39 | Male | Foot |
Of the patients, 14 (46.6%) cases showed >50% improvement in wound size reduction (length × width), eight (26.6%) showed a 30–40% improvement, and six (20%) cases showed a 10–20% improvement in the reduction of the wound size. The remaining two patients showed a <10% reduction in slough.
A selection of case studies showing the pre- and post-debridement outcomes of patients in this study treated with MDT are shown in Figs 1–10. The percentage of wound area reduction in patients is shown in Fig 11. Table 2 details wound size pre- and post treatment, duration of therapy and percentage wound area reduction.
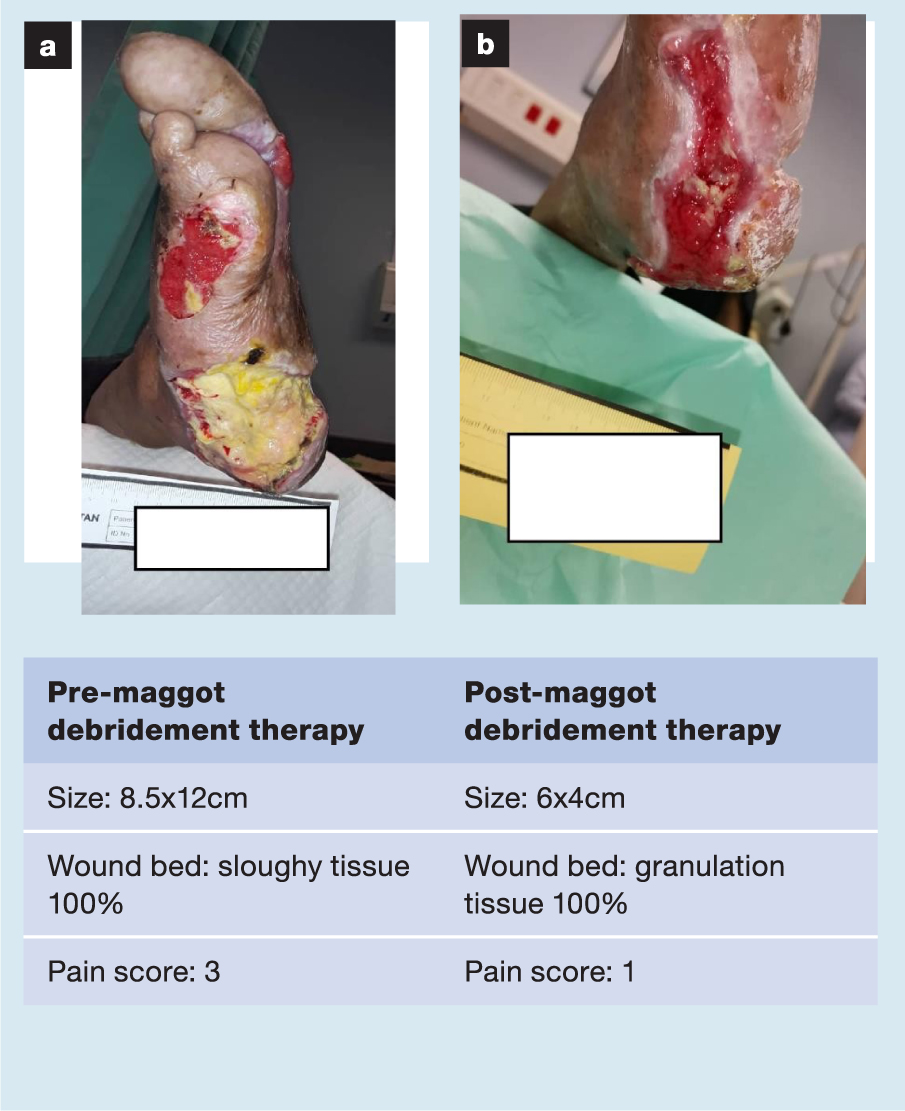
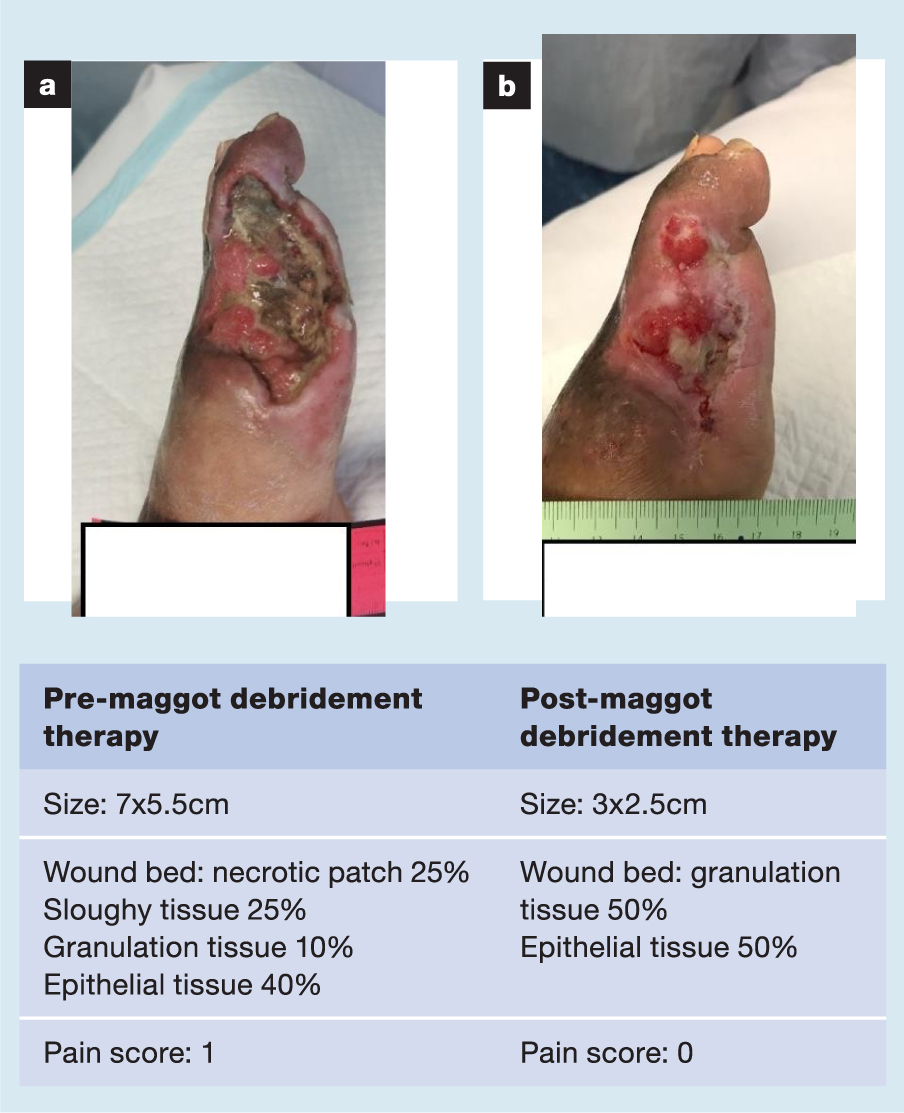
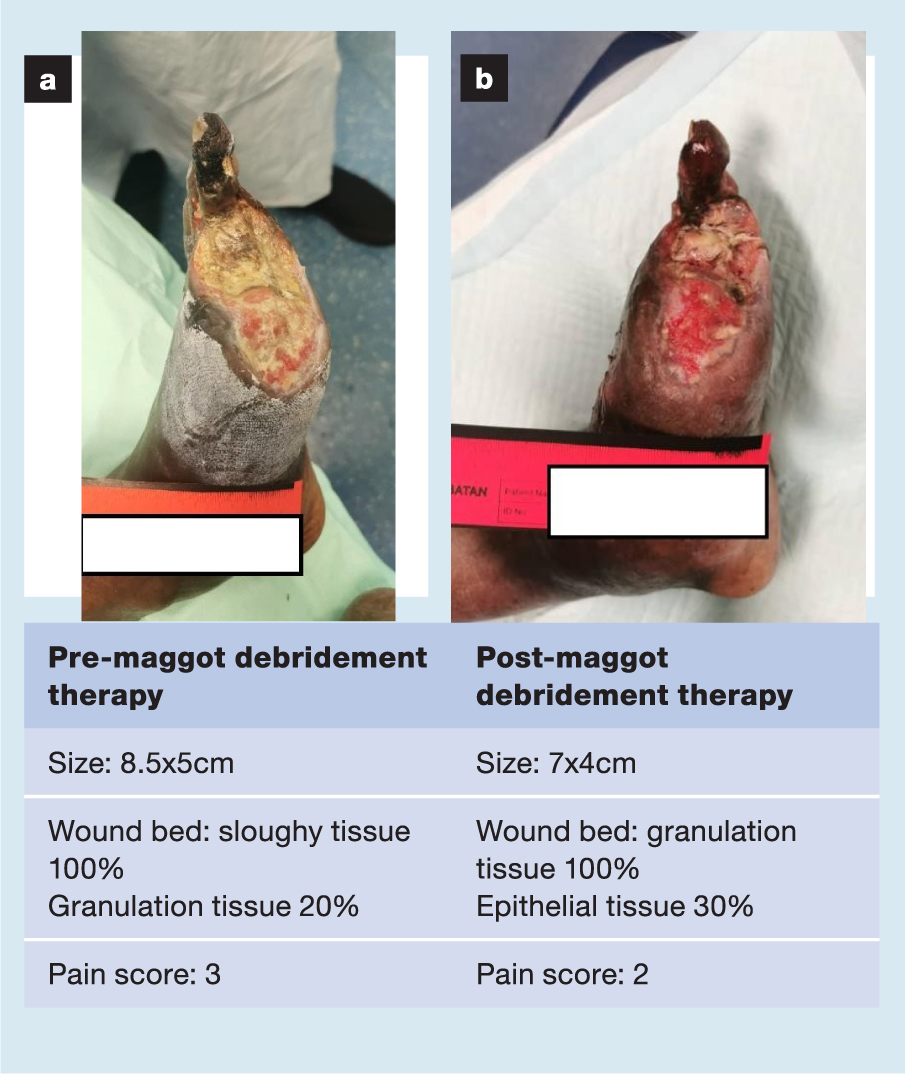
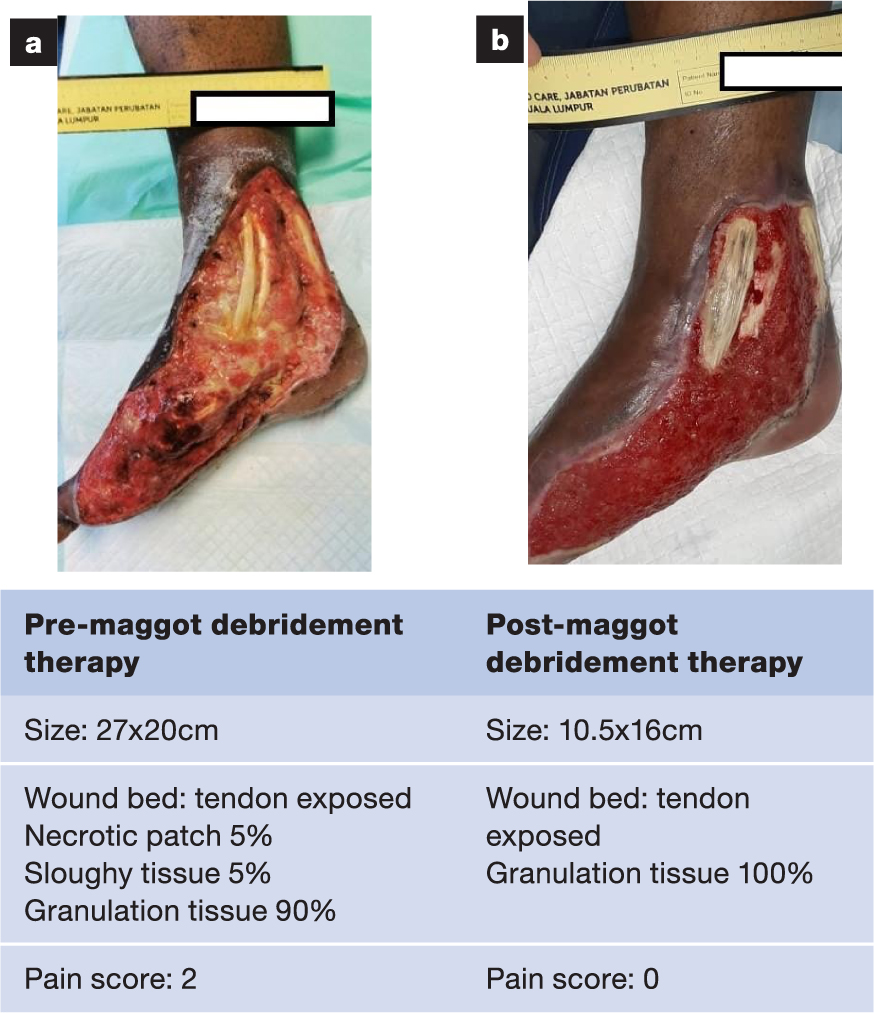
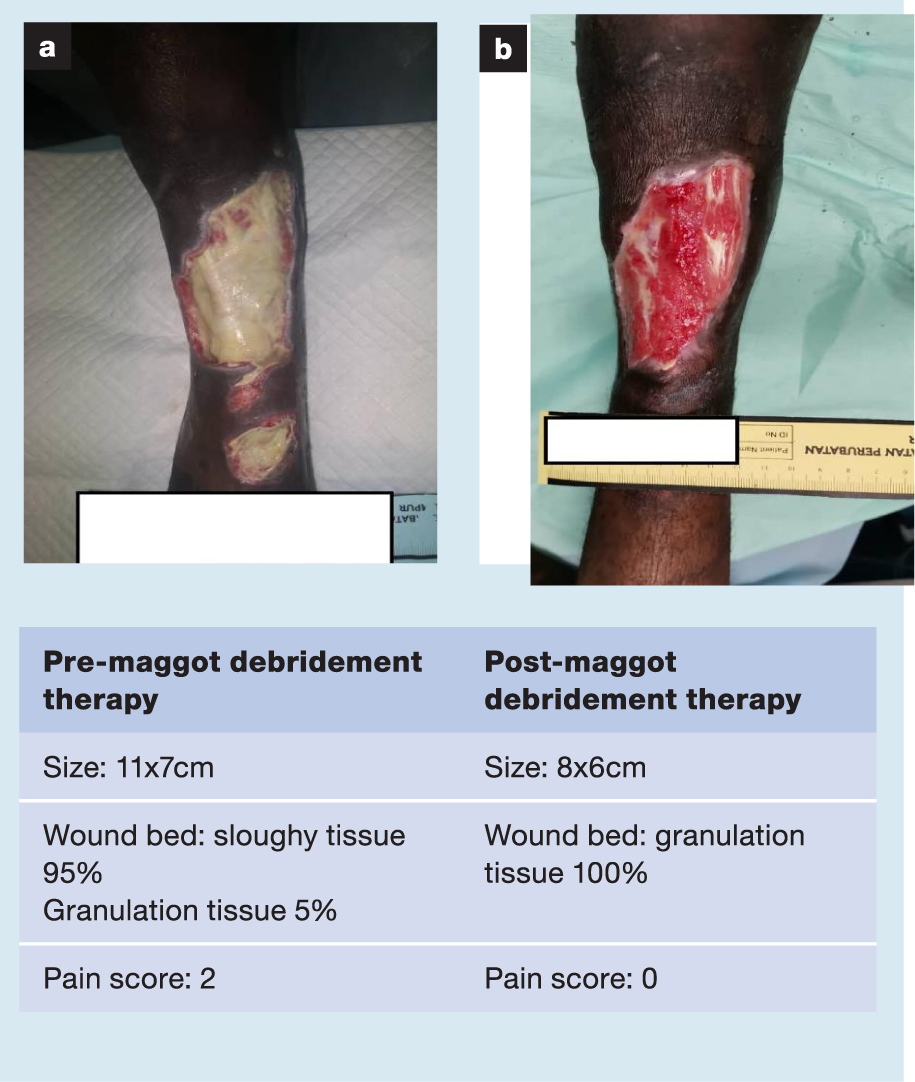
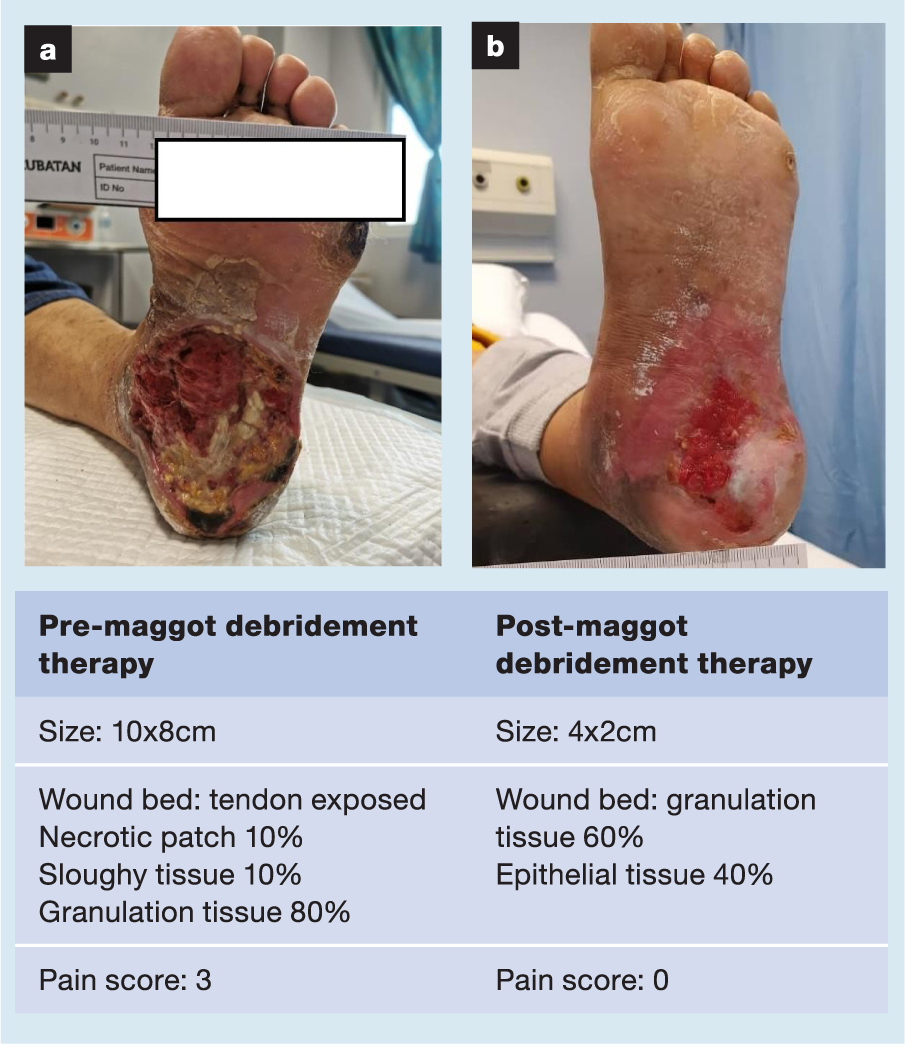
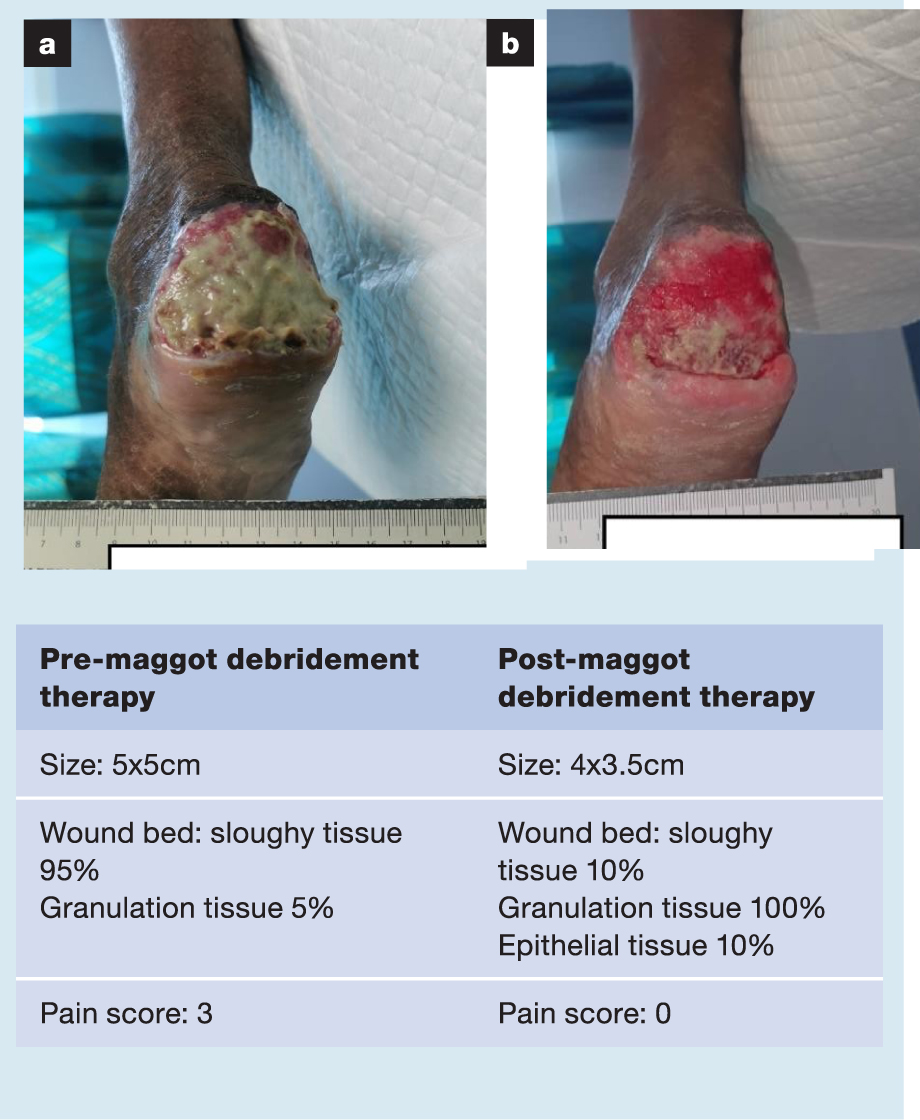
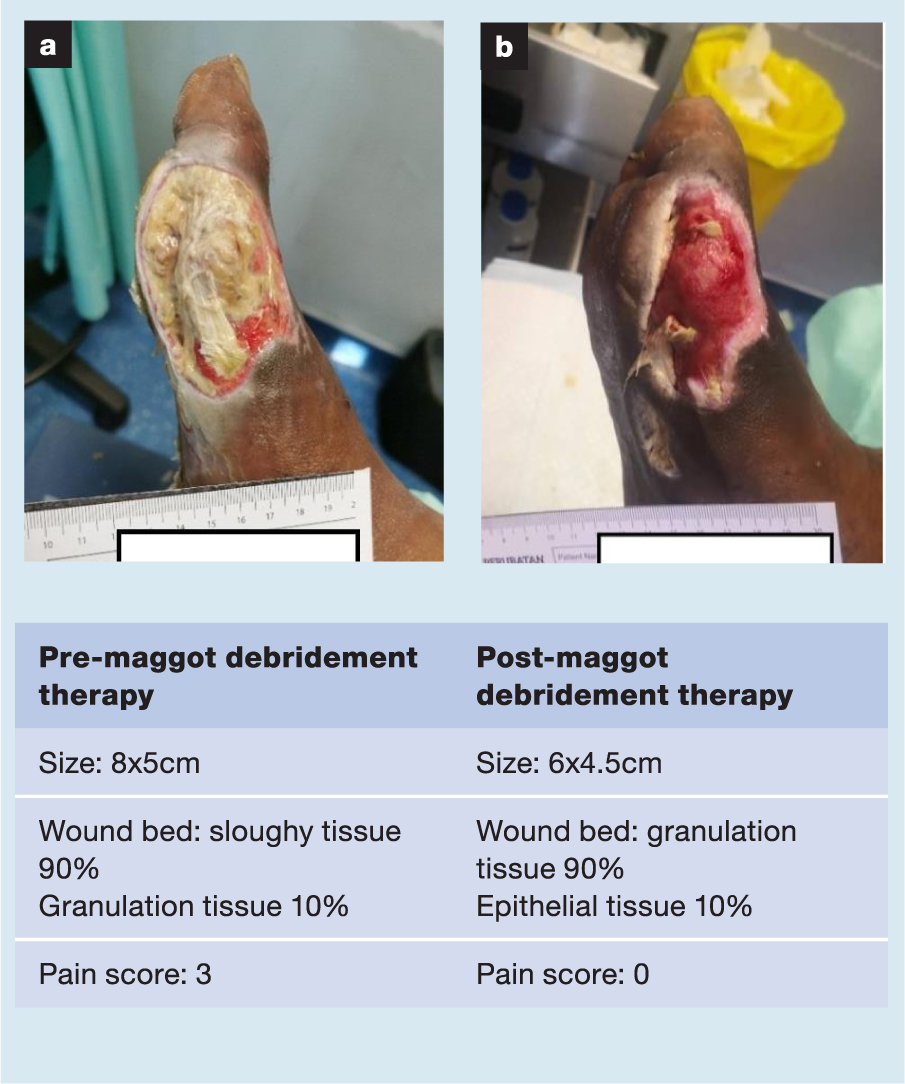
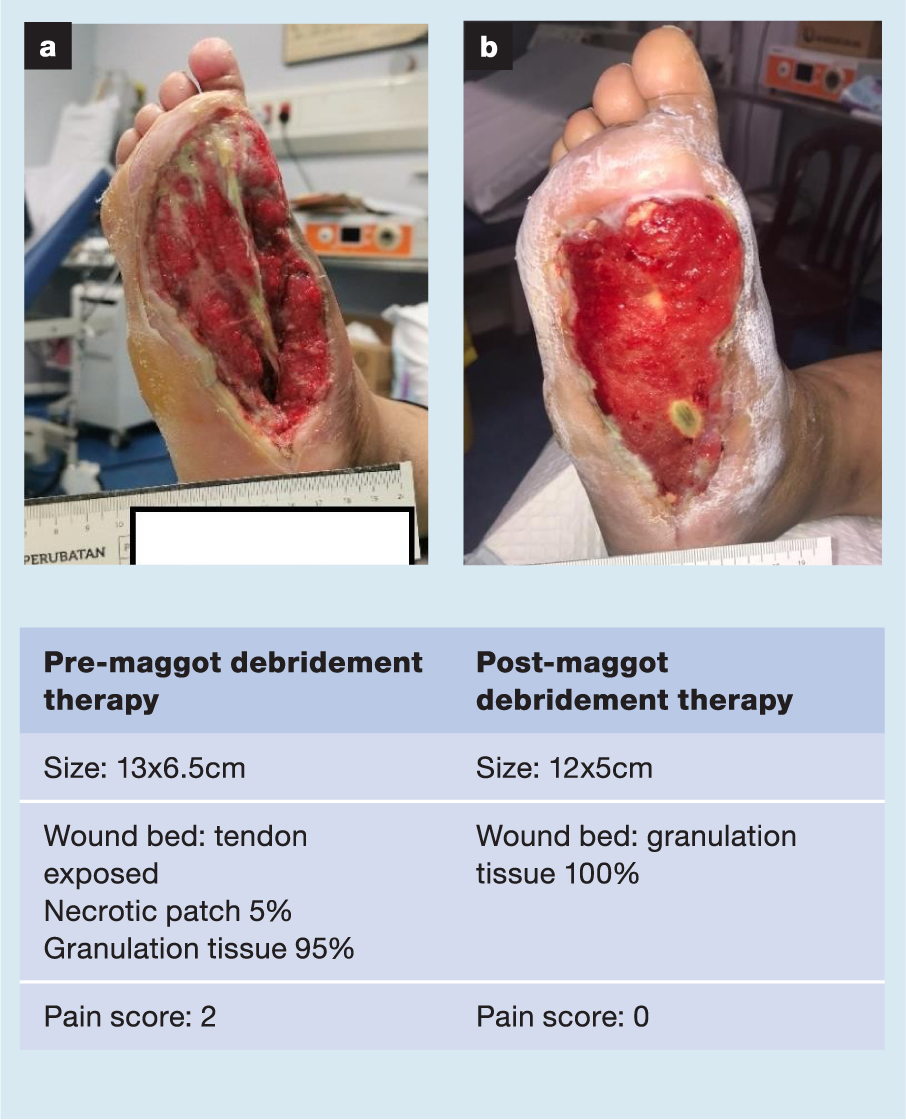
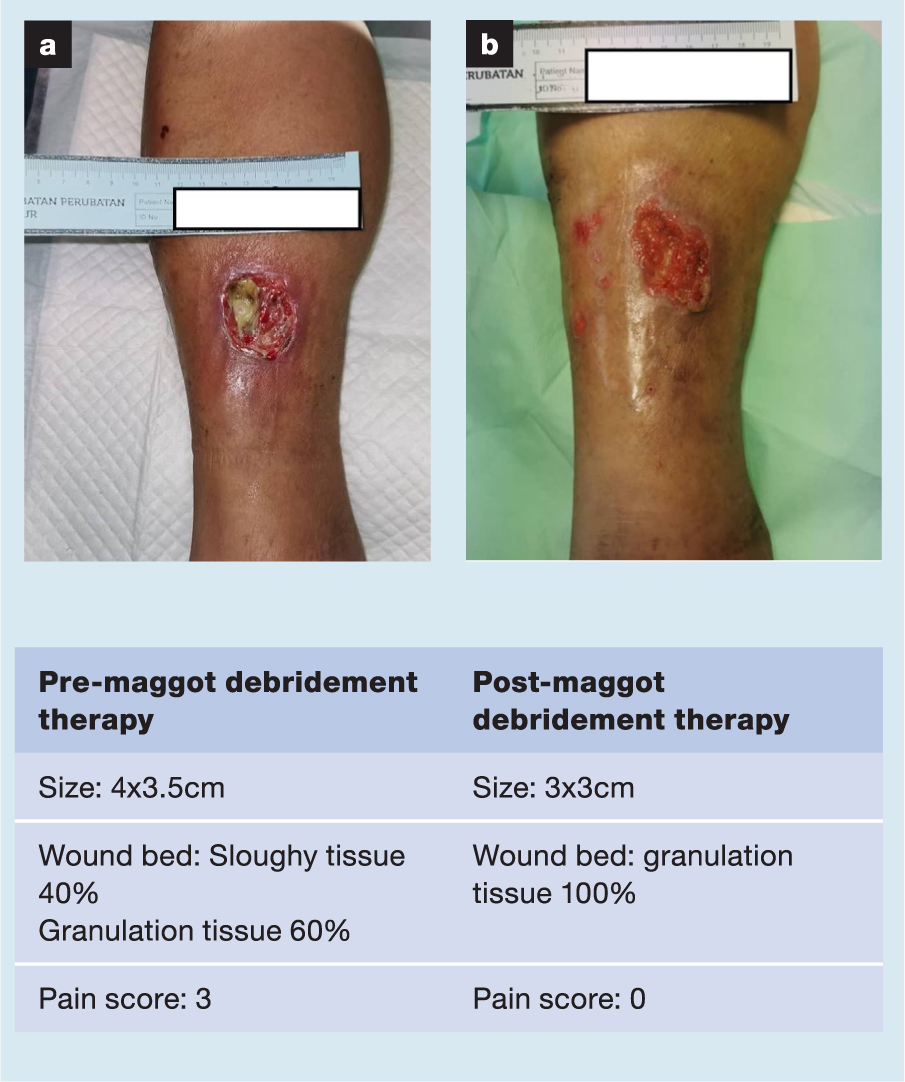
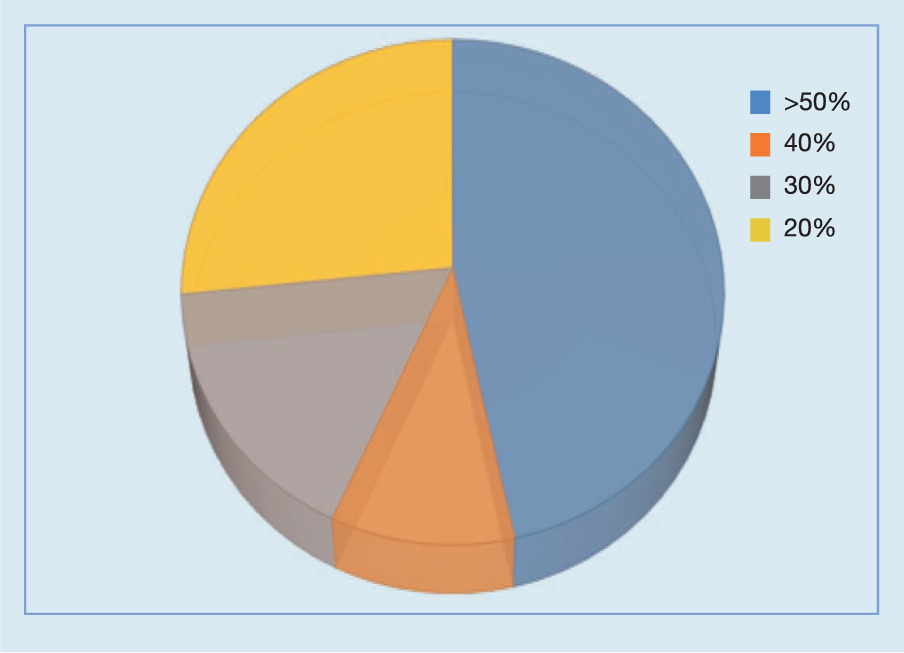
Table 2. Wound size pre- and post treatment, duration of therapy and percentage wound area reduction
| Case number | Pre-treatment wound size, cm | Post-treatment wound size, cm | Wound therapy, start date | Wound therapy, end date | Duration of wound therapy, wound healing, days | Duration of therapy, wound healing, weeks | Wound area reduction, % |
|---|---|---|---|---|---|---|---|
| 1 | 8.5x12 | 6x4 | 3 Jun 20 | 10 Aug 20 | 68 | 10 | 76 |
| 2 | 5.5x5.7 | 5x5.5 | 2 Jun 20 | 5 Jun 20 | 3 | 1 | 12 |
| 3 | 10x5.5 | 7x5 | 15 Jun 20 | 30 Jul 20 | 45 | 6 | 36 |
| 4 | 7.5x6 | 5x3 | 3 Jun 20 | 22 Jun 20 | 19 | 3 | 66 |
| 5 | 8x4.5 | 0x0 | 25 Jun 20 | 2 Oct 20 | 99 | 14 | 100 |
| 6 | 3x8 | 2x6 | 30 Jun 20 | 13 Aug 20 | 44 | 6 | 50 |
| 7 | 7x5 | 4x3 | 17 Jul 20 | 4 Sep 20 | 49 | 7 | 65 |
| 8 | 8.5x5 | 7x4 | 22 Jul 20 | 10 Aug 20 | 19 | 3 | 34 |
| 9 | 12x9 | 9x5 | 7 Jul 20 | 24 Sep 20 | 79 | 11 | 58 |
| 10 | 16x7.5 | 2x2 | 24 Jul 20 | 5 Nov 20 | 132 | 19 | 96 |
| 11 | 7x5.5 | 2x1.5 | 24 Jul 20 | 5 Nov 20 | 104 | 15 | 92 |
| 12 | 27x20 | 10x15 | 23 Jul 20 | 28 Oct 20 | 97 | 14 | 72 |
| 13 | 11x7 | 4x2 | 8 Jul 20 | 10 Dec 20 | 155 | 22 | 89 |
| 14 | 5x3x4 | 3x3x3.5 | 19 Aug 20 | 30 Sep 20 | 42 | 6 | 47 |
| 15 | 14x10 | 12x9 | 18 Sep 20 | 9 Oct 20 | 21 | 3 | 22 |
| 16 | 15x7 | 9x7 | 11 Sep 20 | 13 Oct 20 | 32 | 5 | 40 |
| 17 | 5x4 | 5x4 | 18 Sep 20 | 29 Sep 20 | 11 | 2 | 0 |
| 18 | 6x5 | 3x3 | 27 Oct 20 | 6 Nov 20 | 10 | 2 | 70 |
| 19 | 5x5 | 4x3.5 | 27 Jan 21 | 5 Mar 21 | 37 | 5 | 44 |
| 20 | 10x8 | 4x2 | 18 Jan 21 | 25 Mar 21 | 66 | 9 | 90 |
| 21 | 8x5 | 6x4.5 | 26 Jan 21 | 19 Mar 21 | 52 | 7 | 32 |
| 22 | 13x6.5 | 12x5 | 9 Feb 21 | 22 Mar 21 | 41 | 6 | 30 |
| 23 | 10.3x7 | 10x6.5 | 23 Feb 21 | 8 Mar 21 | 13 | 2 | 10 |
| 24 | 4x3.5 | 3x3 | 23 Feb 21 | 23 Mar 21 | 28 | 4 | 36 |
| 25 | 7x2.5 | 5x2.5 | 22 Feb 21 | 19 Mar 21 | 25 | 4 | 28 |
| 26 | 6.5x5 | 5.5x2.5 | 22 Feb 21 | 19 Mar 21 | 25 | 4 | 58 |
| 27 | 8x2.5 | 4x2 | 23 Feb 21 | 23 Mar 21 | 28 | 4 | 60 |
| 28 | 11x5.5 | 10x5 | 22 Feb 21 | 19 Mar 21 | 25 | 4 | 17 |
| 29 | 12x9 | 11.5x9 | 25 Feb 21 | 23 Mar 21 | 26 | 4 | 4 |
| 30 | 4.5x4 | 4x3.5 | 17 Feb 21 | 8 Mar 21 | 19 | 3 | 22 |
Discussion
It is evident that each patient has a different healing rate. Of the patients in this study, six (20%) required >10 weeks to show improvement in the wound bed; the majority of patients (n=24, 80%) took ≤10 less weeks to achieve an optimum wound bed condition, with <5% slough or necrotic area present at the end of MDT.
Multiple factors may affect the healing rate, for example diabetes control, concurrent peripheral vascular disease, the patient's adherence to treatment, and attendance and nutritional status. These factors are challenging to control in an outpatient setting. From our observation, wound size prior to MDT, however, does not seem to influence the healing rate. As an example, the patient in Case 13 started with a wound of 11×7cm in size, and took 22 weeks of MDT to achieve maximum debridement. On the other hand, the patient in Case 23 started with similar wound size (10.3×7cm); however, in this case it took only two weeks to accomplish maximum debridement.
MDT has been shown to be efficacious in the management of hard-to-heal wounds of various aetiologies, including DFUs, pressure injury, surgical wounds and wounds infected with meticillin-resistant Staphylococcus aureus (MRSA).8,9,10,11,12 In the context of lower limb wounds with underlying diabetes and peripheral vascular disease, MDT has been shown to reduce amputation rates as well as the need for antibiotic therapy.13 Additionally, MDT has been demonstrated to be cost-effective, with one study on venous ulcers estimating the total expenditure on debridement of a wound with MDT (including dressing material and nursing costs) to be less than one fifth of the cost of a wound treated with conventional hydrogel dressings; most likely due to a reduced debridement time and fewer hospital visits.14
A number of proposed mechanisms may account for the improved wound healing observed in patients treated with MDT. Proteolytic enzymes found in the excretory/secretory products of Lucilia sericata larvae have been proposed to aid wound debridement via breakdown of the extracellular matrix.15 These excretory/secretory products have been shown to inhibit the growth of many pathogens responsible for severe soft tissue infections, including MRSA, Pseudomonas aeruginosa and Escherichia coli.16 Ammonium bicarbonate present in maggot excretions may also create an unfavourable environment for bacterial propagation by raising the local pH.17 Bacterial ingestion and destruction by maggots may be another mechanism for disinfection, with one study showing a reduction in the population of Escherichia coli bacteria following ingestion by Lucilia sericata from 67% in the proximal alimentary canal to 18% in the posterior hindgut.18 The excretory/secretory products of larvae may also enhance tissue growth by promoting the proliferation of fibroblasts as well as through the secretion of cytokines.19,20
Our current study has shown maggot debridement therapy to be a safe and tolerable treatment modality, with no adverse effects documented for any of our 30 patients. Although no major complications were associated with the MDT, common patient complaints include minor discomfort, escape of larvae and lack of psychological acceptance.21
Certain wounds are known to be unsuitable for MDT, including dry and desiccated wounds, open wounds in body cavities, wounds in close proximity to vascular structures, and wounds in patients with known allergies to eggs, soybeans or fly larvae.22,23
Limitations
Although this was a case series, limitations to the study include the relatively small sample size, and there was no secondary arm using standard of care, and so no comparisons could be drawn.
Conclusion
In conclusion, we report improvements in wound healing and reductions in wound sizes for DFUs treated with MDT, with no adverse effects and a reduction in wound-related pain over the duration of the treatment. No patients underwent amputation or required inpatient hospital admission for wound-related complications during the period of the study. Further studies comparing MDT to conventional dressings available to Malaysian healthcare facilities would serve to elucidate any advantages of efficacy and cost-effectiveness, and drive wider adoption of MDT in the management of diabetic foot wounds in the Malaysian setting.
Reflective questions
- How effective is utilisation of biotherapy in wound bed preparation?
- How can maggot debridement therapy help reduce pain?
- What, if any, are the complications of using maggot debridement therapy?

